This site is intended for US healthcare professionals only.
From the largest-ever clinical trial in patients with METex14+ with long-term follow-up2*
TEPMETKO (tepotinib) provided robust and lasting responses in
treatment-naïve patients 1-3
ORR in treatment-naïve patients 2+

Durability of response in treatment-naïve patients 1
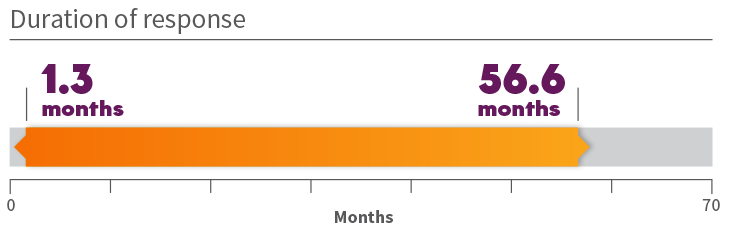

*Long-term follow-up of up to 6 years (range: 0.3-71.9 months). +ORR according to RECIST v1.1 as evaluated by a BIRC.
BIRC=Blinded Independent Review Committee; CI=confidence interval; METex14+=mesenchymal-epithelial transition gene exon 14 skipping alterations; ORR=overall response rate; RECIST=Response Evaluation Criteria in Solid Tumors.
From the largest-ever clinical trial in patients with METex14+ with long-term follow-up2*
TEPMETKO (tepotinib) provided robust and lasting responses in
previously treated patients 1-3
ORR in previously treated patients 2+

Durability of response in previously treated patients 1
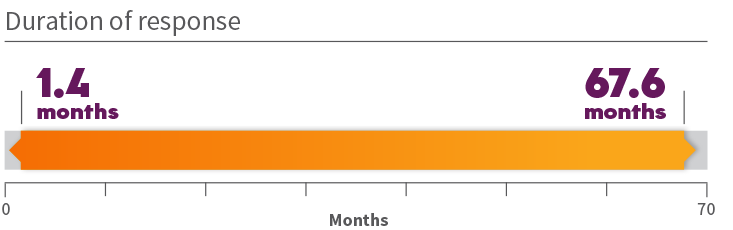

*Long-term follow-up of up to 6 years (range: 0.3-71.9 months). †ORR according to RECIST v1.1 as evaluated by a BIRC.
BIRC=Blinded Independent Review Committee; CI=confidence interval; METex14+=mesenchymal-epithelial transition gene exon 14 skipping alterations; ORR=overall response rate; RECIST=Response Evaluation Criteria in Solid Tumors.
NCCN
PREFERRED
Tepotinib is a National Comprehensive Cancer Network® (NCCN) preferred treatment option in the first-line/subsequent line‡ setting for patients with METex14+ mNSCLC (category 2A)4 § || ¶
‡If MET inhibitors have not previously been given. §Category 2A definition: based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate. ||See the NCCN Guidelines® for detailed recommendations, including other preferred options. ¶The NCCN Guidelines for NSCLC provide recommendations for certain individual biomarkers that should be tested and recommend testing techniques but do not endorse any specific commercially available biomarker assays or commercial laboratories.3
IMPORTANT SAFETY INFORMATION
TEPMETKO can cause interstitial lung disease (ILD)/pneumonitis, which can be fatal. Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis (eg, dyspnea, cough, fever). Immediately withhold TEPMETKO in patients with suspected ILD/pneumonitis and permanently discontinue if no other potential causes of ILD/pneumonitis are identified. ILD/pneumonitis occurred in 2% of patients treated with TEPMETKO, with one patient experiencing a Grade 3 or higher event; this event resulted in death.
TEPMETKO can cause hepatotoxicity, which can be fatal. Monitor liver function tests (including alanine aminotransferase [ALT], aspartate aminotransferase [AST], and total bilirubin) prior to the start of TEPMETKO, every 2 weeks during the first 3 months of treatment, then once a month or as clinically indicated, with more frequent testing in patients who develop increased transaminases or total bilirubin. Based on the severity of the adverse reaction, withhold, dose reduce, or permanently discontinue TEPMETKO. Increased ALT/increased AST occurred in 18% of patients treated with TEPMETKO. Grade 3 or 4 increased ALT/AST occurred in 4.7% of patients. A fatal adverse reaction of hepatic failure occurred in one patient (0.2%). The median time-to-onset of Grade 3 or higher increased ALT/AST was 47 days (range 1 to 262).
TEPMETKO can cause pancreatic toxicity in the form of elevations in amylase and lipase levels. Increased amylase and/or lipase occurred in 13% of patients, with Grade 3 and 4 increases occurring in 5% and 1.2% of patients, respectively. Monitor amylase and lipase levels at baseline and regularly during treatment with TEPMETKO and temporarily withhold, dose reduce, or permanently discontinue based on severity of the adverse event.
TEPMETKO can cause embryo-fetal toxicity. Based on findings in animal studies and its mechanism of action, TEPMETKO can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential or males with female partners of reproductive potential to use effective contraception during treatment with TEPMETKO and for one week after the last dose.
Avoid concomitant use of TEPMETKO with certain P-gp substrates where minimal concentration changes may lead to serious or life-threatening toxicities. If concomitant use is unavoidable, reduce the P-gp substrate dosage if recommended in its approved product labeling.
Fatal adverse reactions occurred in one patient (0.3%) due to pneumonitis, one patient (0.3%) due to hepatic failure, one patient (0.3%) due to dyspnea from fluid overload, one patient (0.3%) due to pneumonia, one patient (0.3%) due to sepsis, and one patient (0.3%) from unknown cause.
Serious adverse reactions occurred in 51% of patients who received TEPMETKO. Serious adverse reactions in >2% of patients included pleural effusion (6%), pneumonia (6%), edema (5%), general health deterioration (3.8%), dyspnea (3.5%), musculoskeletal pain (2.9%), and pulmonary embolism (2.2%).
The most common adverse reactions (≥20%) in patients who received TEPMETKO were edema (81%), nausea (31%), fatigue (30%), musculoskeletal pain (30%), diarrhea (29%), dyspnea (24%), rash (21%), and decreased appetite (21%).
Clinically relevant adverse reactions in <10% of patients who received TEPMETKO included ILD/pneumonitis, fever, dizziness, pruritus, and headache.
Selected laboratory abnormalities (≥20%) from baseline in patients receiving TEPMETKO in descending order were: decreased albumin (81%), increased creatinine (60%), decreased lymphocytes (57%), increased alkaline phosphatase (ALP) (52%), increased ALT (50%), increased AST (40%), decreased sodium (36%), decreased hemoglobin (31%), increased gamma-glutamyltransferase (GGT) (29%), increased potassium (26%), increased amylase (25%), decreased leukocytes (25%), decreased platelets (24%), and increased lipase (21%).
The most common Grade 3-4 laboratory abnormalities (≥2%) in descending order were: decreased lymphocytes (15%), decreased albumin (9%), decreased sodium (9%), increased GGT (6%), increased amylase (5%), increased lipase (5%), increased ALT (4.9%), increased AST (3.6%), and decreased hemoglobin (3.6%).
INDICATION
TEPMETKO is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) harboring mesenchymal-epithelial transition (MET) exon 14 skipping alterations.
Please see the full Prescribing Information for TEPMETKO.
References: 1. TEPMETKO® (tepotinib) [prescribing information]. EMD Serono, Inc., Boston, MA. 2. Mazieres J, Paik PK, Garassino MC, et al. Tepotinib treatment in patients with MET exon 14–skipping non–small cell lung cancer: long-term follow-up of the VISION phase 2 nonrandomized clinical trial. JAMA Oncol. 2023;9(9):1260-1266. doi:10.1001/jamaoncol.2023.1962 3. Paik PK, Felip E, Veillon R, et al. Tepotinib in non–small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931-943. doi:10.1056/NEJMoa2004407 4. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed February 27, 2024. To view the most recent and complete version of the guidelines, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.