This site is intended for US healthcare professionals only.
The largest trial in patients with METex14+ mNSCLC1
Trial design
VISION: A single-arm, open-label, multicenter, non-randomized, multicohort study1,2
The efficacy population included 164 treatment-naïve patients and 149 previously treated patients.1,2
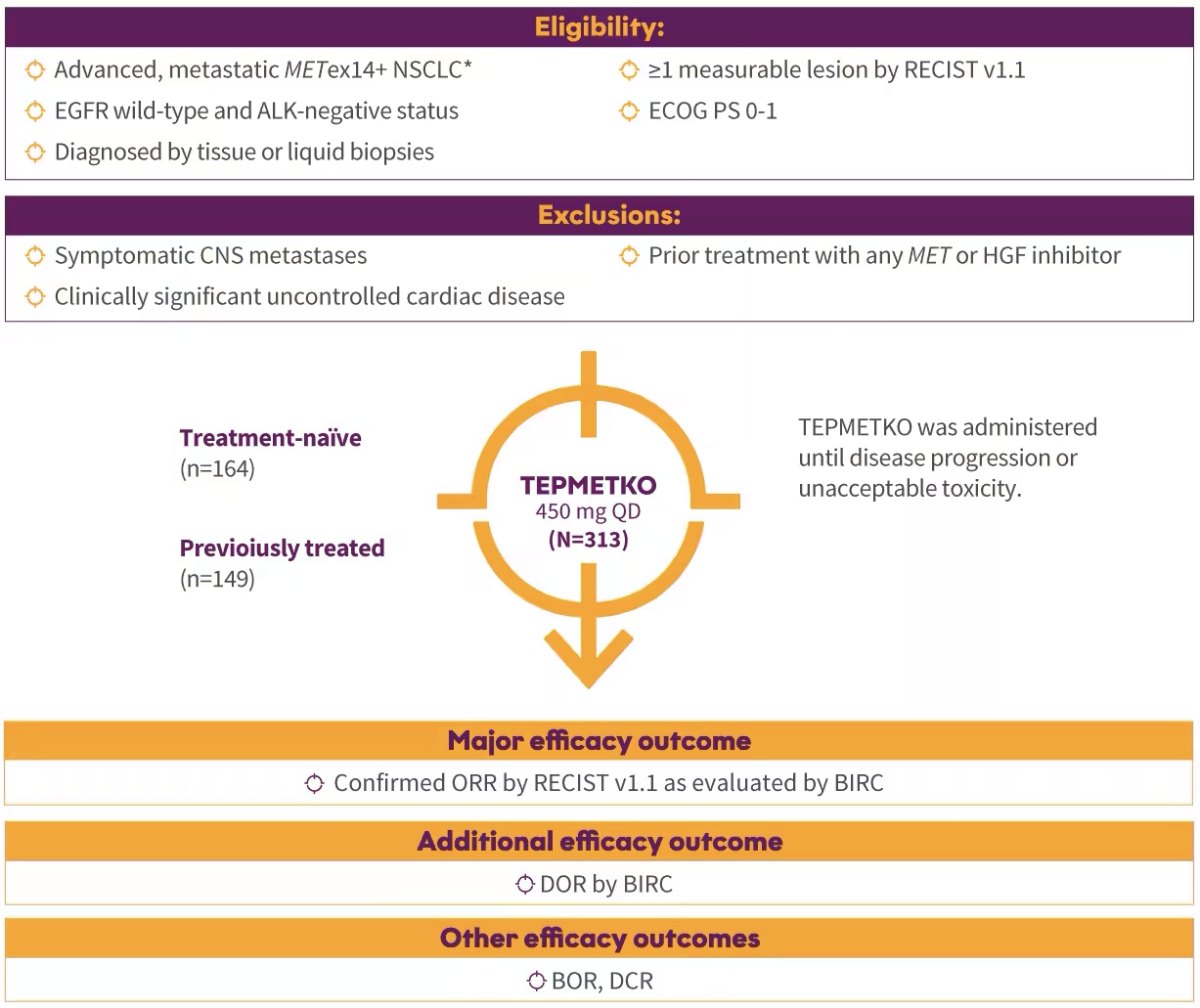
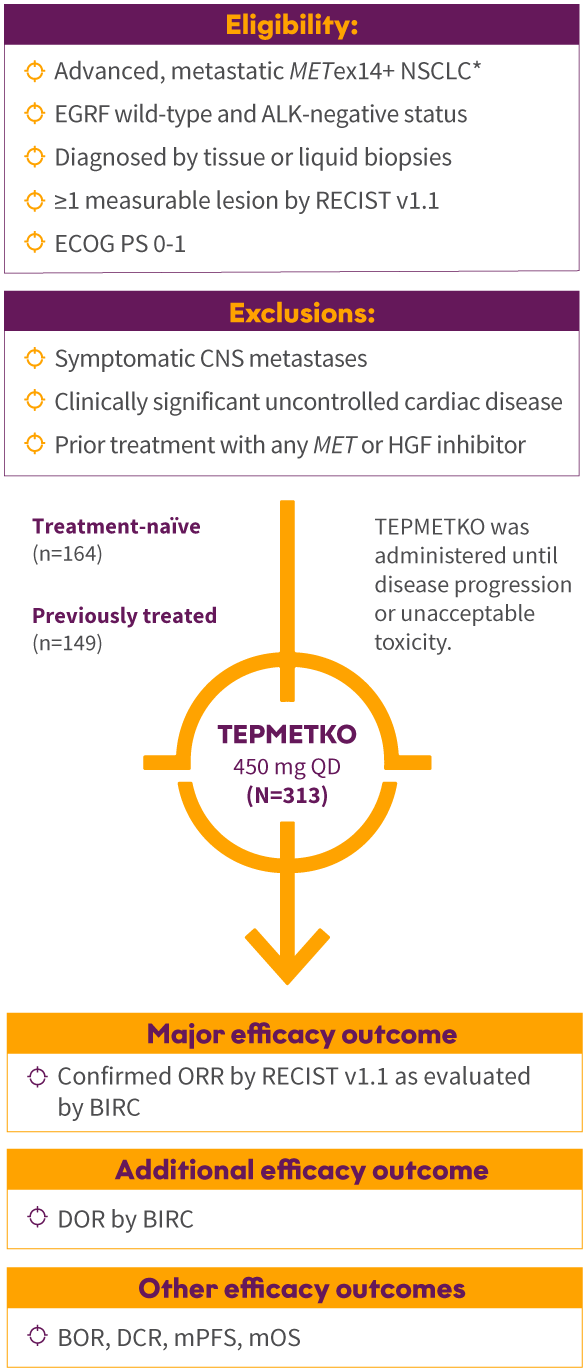
*Identification of METex14 skipping alterations was prospectively determined using central laboratories employing either a PCR-based or next-generation sequencing-based clinical trial assay using tissue (66%) and/or plasma (57%) samples. An FDA-approved test for detection of METex14 skipping alterations in NSCLC for selecting patients for treatment with TEPMETKO is not available.
Patient characteristics
Patients studied in the VISION Trial (N=313)1-3
Disease characteristics 81% had adenocarcinoma histology† 13% had CNS metastases | Age/ECOG status Median age of 72 years |
Line of therapy 52% first line (n=164) 48% previously treated (n=149)‡ – 84% prior platinum-based therapy – 54% immune-based therapy | Race and gender 62% White 34% Asian 49% male 51% female | Smoking status 49% never smokers 51% former smokers
|
Disease characteristics 81% had adenocarcinoma histology† 13% had CNS metastases |
Age/ECOG status Median age of 72 years 79% were ≥65 41% were ≥75 26% had ECOG Performance Status (PS) 0 and 74% had ECOG PS 1 |
Line of therapy 52% first line (n=164) 48% previously treated (n=149)‡ – 84% prior platinum-based therapy – 54% immune-based therapy |
Race and Gender 62% White 34% Asian 49% male 51% female |
Smoking status 49% never smokers 51% former smokers |
†Other histological subtypes in Cohorts A and C included squamous and sarcomatoid histology.
‡Had progressed on up to 2 lines of prior systemic therapies.
METex14 skipping alterations were identified through either PCR or next-generation sequencing testing1§

- 66% of patients by tissue (RNA-based) testing
- 57% of patients by plasma (ctDNA-based) testing
§Some patients tested positive using both methodologies.
ALK=anaplastic lymphoma kinase; BIRC=Blinded Independent Review Committee; BOR=best overall response; CNS=central nervous system; ctDNA=circulating tumor deoxyribonucleic acid; DCR=disease control rate; DOR=duration of response; ECOG PS=Eastern Cooperative Oncology Group Performance Status; EGFR=epidermal growth factor receptor; HGF=hepatocyte growth factor; MET=mesenchymal-epithelial transition; METex14+=mesenchymal-epithelial transition exon 14 skipping alterations; mNSCLC=metastatic non-small cell lung cancer; NGS=next-generation sequencing; NSCLC=non-small cell lung cancer; ORR=overall response rate; PCR=polymerase chain reaction; QD=once daily; RECIST=Response Evaluation Criteria in Solid Tumors; RNA=ribonucleic acid.
IMPORTANT SAFETY INFORMATION
TEPMETKO can cause interstitial lung disease (ILD)/pneumonitis, which can be fatal. Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis (eg, dyspnea, cough, fever). Immediately withhold TEPMETKO in patients with suspected ILD/pneumonitis and permanently discontinue if no other potential causes of ILD/pneumonitis are identified. ILD/pneumonitis occurred in 2% of patients treated with TEPMETKO, with one patient experiencing a Grade 3 or higher event; this event resulted in death.
TEPMETKO can cause hepatotoxicity, which can be fatal. Monitor liver function tests (including alanine aminotransferase [ALT], aspartate aminotransferase [AST], and total bilirubin) prior to the start of TEPMETKO, every 2 weeks during the first 3 months of treatment, then once a month or as clinically indicated, with more frequent testing in patients who develop increased transaminases or total bilirubin. Based on the severity of the adverse reaction, withhold, dose reduce, or permanently discontinue TEPMETKO. Increased ALT/increased AST occurred in 18% of patients treated with TEPMETKO. Grade 3 or 4 increased ALT/AST occurred in 4.7% of patients. A fatal adverse reaction of hepatic failure occurred in one patient (0.2%). The median time-to-onset of Grade 3 or higher increased ALT/AST was 47 days (range 1 to 262).
TEPMETKO can cause pancreatic toxicity in the form of elevations in amylase and lipase levels. Increased amylase and/or lipase occurred in 13% of patients, with Grade 3 and 4 increases occurring in 5% and 1.2% of patients, respectively. Monitor amylase and lipase levels at baseline and regularly during treatment with TEPMETKO and temporarily withhold, dose reduce, or permanently discontinue based on severity of the adverse event.
TEPMETKO can cause embryo-fetal toxicity. Based on findings in animal studies and its mechanism of action, TEPMETKO can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential or males with female partners of reproductive potential to use effective contraception during treatment with TEPMETKO and for one week after the last dose.
Avoid concomitant use of TEPMETKO with certain P-gp substrates where minimal concentration changes may lead to serious or life-threatening toxicities. If concomitant use is unavoidable, reduce the P-gp substrate dosage if recommended in its approved product labeling.
Fatal adverse reactions occurred in one patient (0.3%) due to pneumonitis, one patient (0.3%) due to hepatic failure, one patient (0.3%) due to dyspnea from fluid overload, one patient (0.3%) due to pneumonia, one patient (0.3%) due to sepsis, and one patient (0.3%) from unknown cause.
Serious adverse reactions occurred in 51% of patients who received TEPMETKO. Serious adverse reactions in >2% of patients included pleural effusion (6%), pneumonia (6%), edema (5%), general health deterioration (3.8%), dyspnea (3.5%), musculoskeletal pain (2.9%), and pulmonary embolism (2.2%).
The most common adverse reactions (≥20%) in patients who received TEPMETKO were edema (81%), nausea (31%), fatigue (30%), musculoskeletal pain (30%), diarrhea (29%), dyspnea (24%), rash (21%), and decreased appetite (21%).
Clinically relevant adverse reactions in <10% of patients who received TEPMETKO included ILD/pneumonitis, fever, dizziness, pruritus, and headache.
Selected laboratory abnormalities (≥20%) from baseline in patients receiving TEPMETKO in descending order were: decreased albumin (81%), increased creatinine (60%), decreased lymphocytes (57%), increased alkaline phosphatase (ALP) (52%), increased ALT (50%), increased AST (40%), decreased sodium (36%), decreased hemoglobin (31%), increased gamma-glutamyltransferase (GGT) (29%), increased potassium (26%), increased amylase (25%), decreased leukocytes (25%), decreased platelets (24%), and increased lipase (21%).
The most common Grade 3-4 laboratory abnormalities (≥2%) in descending order were: decreased lymphocytes (15%), decreased albumin (9%), decreased sodium (9%), increased GGT (6%), increased amylase (5%), increased lipase (5%), increased ALT (4.9%), increased AST (3.6%), and decreased hemoglobin (3.6%).
INDICATION
TEPMETKO is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) harboring mesenchymal-epithelial transition (MET) exon 14 skipping alterations.
Please see the full Prescribing Information for TEPMETKO.
References: 1. TEPMETKO® (tepotinib) [prescribing information]. EMD Serono, Inc., Boston, MA. 2. Mazieres J, Paik PK, Garassino MC, et al. Tepotinib treatment in patients with MET exon 14–skipping non–small cell lung cancer: long-term follow-up of the VISION phase 2 nonrandomized clinical trial. JAMA Oncol. 2023;9(9):1260-1266. doi:10.1001/jamaoncol.2023.1962 3. Smit EF, Garassino MC, Felip E, et al. Tepotinib outcomes according to prior therapies in patients with MET exon 14 (METex14) skipping NSCLC. Poster presented at: European Society for Medical Oncology (ESMO) congress; September 9-13, 2022; Paris, France. Poster 985P.