This site is intended for US healthcare professionals only.
Safety profile1
Warnings & Precautions
The safety profile of TEPMETKO® (tepotinib) reflects exposure to TEPMETKO in 506 patients with various solid tumors. These patients were enrolled in 5 open-label, single-arm studies, in which they received TEPMETKO as a single agent at a dose of 450 mg once daily. This included 313 patients with NSCLC positive for METex14 skipping alterations who received TEPMETKO in VISION.
Interstitial lung disease (ILD)/Pneumonitis
ILD/pneumonitis, which can be fatal, occurred in patients treated with TEPMETKO. ILD/pneumonitis occurred in 2% patients treated with TEPMETKO, with one patient experiencing a Grade 3 or higher event; this event resulted in death. Five patients (1%) discontinued TEPMETKO due to ILD/pneumonitis.
Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis (e.g., dyspnea, cough, fever). Immediately withhold TEPMETKO in patients with suspected ILD/pneumonitis and permanently discontinue if no other potential causes of ILD/pneumonitis are identified.
Hepatotoxicity
Hepatotoxicity occurred in patients treated with TEPMETKO. Increased alanine aminotransferase (ALT)/increased aspartate aminotransferase (AST) occurred in 18% of patients treated with TEPMETKO. Grade 3 or 4 increased ALT/AST occurred in 4.7% of patients. A fatal adverse reaction of hepatic failure occurred in one patient (0.2%). Four patients (0.8%) discontinued TEPMETKO due to increased ALT/AST. The median time-to-onset of Grade 3 or higher increased ALT/AST was 47 days (range 1 to 262).
Monitor liver function tests (including ALT, AST, and total bilirubin) prior to the start of TEPMETKO, every 2 weeks during the first 3 months of treatment, then once a month or as clinically indicated, with more frequent testing in patients who develop increased transaminases or bilirubin. Based on the severity of the adverse reaction, withhold, dose reduce, or permanently discontinue TEPMETKO.
Pancreatic Toxicity
Elevations in amylase and lipase levels occurred in patients treated with TEPMETKO. Increased amylase and/or lipase occurred in 13% of patients treated with TEPMETKO. Grade 3 and 4 increased amylase and/or lipase occurred in 5% and 1.2% of patients, respectively. Monitor amylase and lipase at baseline and regularly during treatment with TEPMETKO. Based on the severity of the adverse drug reaction, temporarily withhold, dose reduce, or permanently discontinue TEPMETKO.
Embryo-fetal toxicity
Based on findings in animal studies and its mechanism of action TEPMETKO can cause fetal harm when administered to a pregnant woman. Oral administration of tepotinib to pregnant rabbits during the period of organogenesis resulted in malformations (teratogenicity) and anomalies at exposures less than the human exposure based on area under the curve (AUC) at the 450 mg daily clinical dose. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential or males with female partners of reproductive potential to use effective contraception during treatment with TEPMETKO and for one week after the last dose.
Adverse reactions
The safety and tolerability profile of TEPMETKO was studied in 313 patients in the VISION Trial1
- Fatal adverse reactions occurred in 1.9% of patients who received TEPMETKO, including pneumonitis (0.3%), hepatic failure (0.3%), dyspnea from fluid overload (0.3%), pneumonia (0.3%), sepsis (0.3%), and death of unknown cause (0.3%)
- Serious adverse reactions occurred in 51% of patients who received TEPMETKO. Serious adverse reactions in > 2% of patients included pleural effusion (6%), pneumonia (6%), edema (5%), general health deterioration (3.8%), dyspnea (3.5%), musculoskeletal pain (2.9%), and pulmonary embolism (2.2%)
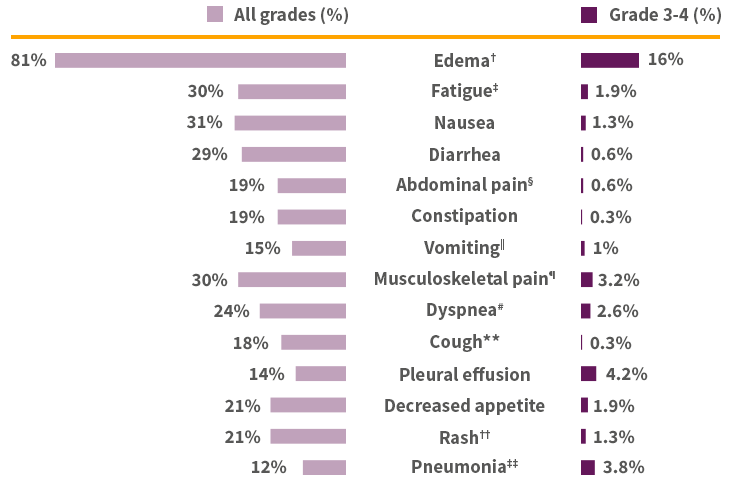
Adverse reactions in ≥10% of patients with NSCLC harboring METex14+1*

Due to an AR in patients who received TEPMETKO1
- Permanent discontinuation – 25%
- The most frequent ARs (>1%) leading to permanent discontinuations of TEPMETKO were edema (8%), pleural effusion (1.6%), and general health deterioration (1.6%)
- Dosage interruptions – 53%
- ARs which required dosage interruption in >2% of patients who received TEPMETKO included edema (28%), increased blood creatinine (6%), pleural effusion (3.5%), nausea (3.2%), increased ALT (2.9%), pneumonia (2.6%), decreased appetite (2.2%), and dyspnea (2.2%)
- Dose reductions – 36%
- ARs which required dose reductions in >2% of patients who received TEPMETKO included edema (22%), increased blood creatinine (2.9%), fatigue (2.2%), and pleural effusion (2.2%)
- ARs which required dose reductions in >2% of patients who received TEPMETKO included edema (22%), increased blood creatinine (2.9%), fatigue (2.2%), and pleural effusion (2.2%)
NSCLC=non-small cell lung cancer.
Laboratory abnormalities1*§§
- Selected laboratory abnormalities (≥20%) from baseline in patients receiving TEPMETKO in descending order were: decreased albumin (81%), increased creatinine (60%), decreased lymphocytes (57%), increased ALP (52%), increased ALT (50%), increased AST (40%), decreased sodium (36%), decreased hemoglobin (31%), increased GGT (29%), increased potassium (26%), increased amylase (25%), decreased leukocytes (25%), decreased platelets (24%), and increased lipase (21%)
- The most common Grade 3-4 laboratory abnormalities (≥2%) in descending order were: decreased lymphocytes (15%), decreased albumin (9%), decreased sodium (9%), increased GGT (6%), increased amylase (5%), increased lipase (5%), increased ALT (4.9%), increased AST (3.6%), decreased hemoglobin (3.6%)
TEPMETKO has no known contraindications.1
*Severity as defined by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03. †Edema includes eye edema, face edema, generalized edema, localized edema, edema, genital edema, peripheral edema, peripheral swelling, periorbital edema, and scrotal edema. ‡Fatigue includes asthenia and fatigue. §Abdominal pain includes abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, gastrointestinal pain, and hepatic pain. ‖Vomiting includes retching and vomiting. ¶Musculoskeletal pain includes arthralgia, arthritis, back pain, bone pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, non-cardiac chest pain, pain in extremity, and spinal pain. #Dyspnea includes dyspnea, dyspnea at rest, and dyspnea exertional. **Cough includes cough, and productive cough. ††Rash includes rash, palmar-plantar erythrodysaesthesia syndrome, rash maculo-papular, eczema, exfoliative rash, rash erythematous, rash pustular, skin exfoliation, dermatitis acneiform, drug eruption, dermatitis, rash pruritic, dermatitis bullous, toxic skin eruption. ‡‡Pneumonia includes pneumonia, pneumonia aspiration, and pneumonia bacterial. §§The denominator used to calculate the rate varied from 268 to 309 based on the number of patients with a baseline value and at least one post-treatment value.
Peripheral edema, a class effect of MET inhibitors, was the most common TRAE observed during the trial, but its underlying mechanism remains unknown
Proactive monitoring for peripheral edema is recommended and can be managed with dose reduction of TEPMETKO® (tepotinib) or temporary discontinuation.
Compensatory management of peripheral edema includes:
ALT=alanine aminotransferase; METex14=mesenchymal-epithelial transition exon 14; NSCLC=non-small cell lung cancer; TRAE=treatment-related adverse event.
IMPORTANT SAFETY INFORMATION
TEPMETKO can cause interstitial lung disease (ILD)/pneumonitis, which can be fatal. Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis (eg, dyspnea, cough, fever). Immediately withhold TEPMETKO in patients with suspected ILD/pneumonitis and permanently discontinue if no other potential causes of ILD/pneumonitis are identified. ILD/pneumonitis occurred in 2% of patients treated with TEPMETKO, with one patient experiencing a Grade 3 or higher event; this event resulted in death.
TEPMETKO can cause hepatotoxicity, which can be fatal. Monitor liver function tests (including alanine aminotransferase [ALT], aspartate aminotransferase [AST], and total bilirubin) prior to the start of TEPMETKO, every 2 weeks during the first 3 months of treatment, then once a month or as clinically indicated, with more frequent testing in patients who develop increased transaminases or total bilirubin. Based on the severity of the adverse reaction, withhold, dose reduce, or permanently discontinue TEPMETKO. Increased ALT/increased AST occurred in 18% of patients treated with TEPMETKO. Grade 3 or 4 increased ALT/AST occurred in 4.7% of patients. A fatal adverse reaction of hepatic failure occurred in one patient (0.2%). The median time-to-onset of Grade 3 or higher increased ALT/AST was 47 days (range 1 to 262).
TEPMETKO can cause pancreatic toxicity in the form of elevations in amylase and lipase levels. Increased amylase and/or lipase occurred in 13% of patients, with Grade 3 and 4 increases occurring in 5% and 1.2% of patients, respectively. Monitor amylase and lipase levels at baseline and regularly during treatment with TEPMETKO and temporarily withhold, dose reduce, or permanently discontinue based on severity of the adverse event.
TEPMETKO can cause embryo-fetal toxicity. Based on findings in animal studies and its mechanism of action, TEPMETKO can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential or males with female partners of reproductive potential to use effective contraception during treatment with TEPMETKO and for one week after the last dose.
Avoid concomitant use of TEPMETKO with certain P-gp substrates where minimal concentration changes may lead to serious or life-threatening toxicities. If concomitant use is unavoidable, reduce the P-gp substrate dosage if recommended in its approved product labeling.
Fatal adverse reactions occurred in one patient (0.3%) due to pneumonitis, one patient (0.3%) due to hepatic failure, one patient (0.3%) due to dyspnea from fluid overload, one patient (0.3%) due to pneumonia, one patient (0.3%) due to sepsis, and one patient (0.3%) from unknown cause.
Serious adverse reactions occurred in 51% of patients who received TEPMETKO. Serious adverse reactions in >2% of patients included pleural effusion (6%), pneumonia (6%), edema (5%), general health deterioration (3.8%), dyspnea (3.5%), musculoskeletal pain (2.9%), and pulmonary embolism (2.2%).
The most common adverse reactions (≥20%) in patients who received TEPMETKO were edema (81%), nausea (31%), fatigue (30%), musculoskeletal pain (30%), diarrhea (29%), dyspnea (24%), rash (21%), and decreased appetite (21%).
Clinically relevant adverse reactions in <10% of patients who received TEPMETKO included ILD/pneumonitis, fever, dizziness, pruritus, and headache.
Selected laboratory abnormalities (≥20%) from baseline in patients receiving TEPMETKO in descending order were: decreased albumin (81%), increased creatinine (60%), decreased lymphocytes (57%), increased alkaline phosphatase (ALP) (52%), increased ALT (50%), increased AST (40%), decreased sodium (36%), decreased hemoglobin (31%), increased gamma-glutamyltransferase (GGT) (29%), increased potassium (26%), increased amylase (25%), decreased leukocytes (25%), decreased platelets (24%), and increased lipase (21%).
The most common Grade 3-4 laboratory abnormalities (≥2%) in descending order were: decreased lymphocytes (15%), decreased albumin (9%), decreased sodium (9%), increased GGT (6%), increased amylase (5%), increased lipase (5%), increased ALT (4.9%), increased AST (3.6%), and decreased hemoglobin (3.6%).
INDICATION
TEPMETKO is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) harboring mesenchymal-epithelial transition (MET) exon 14 skipping alterations.
Please see the full Prescribing Information for TEPMETKO.
References: 1. TEPMETKO® (tepotinib) [prescribing information]. EMD Serono, Inc., Boston, MA. 2. Mazieres J, Paik PK, Garassino MC, et al. Tepotinib treatment in patients with MET exon 14–skipping non–small cell lung cancer: long-term follow-up of the VISION phase 2 nonrandomized clinical trial. JAMA Oncol. 2023;9(9)(suppl 2):1-15. doi:10.1001/jamaoncol.2023.1962.