This site is intended for US healthcare professionals only.
For all eligible patients with METex14+ mNSCLC, consider
TEPMETKO® (tepotinib)—The only approved once-daily oral MET inhibitor1
Convenient once-daily dosing
Recommended Starting Dosage: 450 mg (Two 225 mg tablets)
Advise patients to:
- Take their dose at approximately the same time every day until disease progression or unacceptable toxicity
- Swallow tablets whole. Do not chew, crush, or split tablets.
- Never make up a missed dose within 8 hours of the next scheduled dose. If vomiting occurs after taking a dose, take the next dose at the scheduled time.
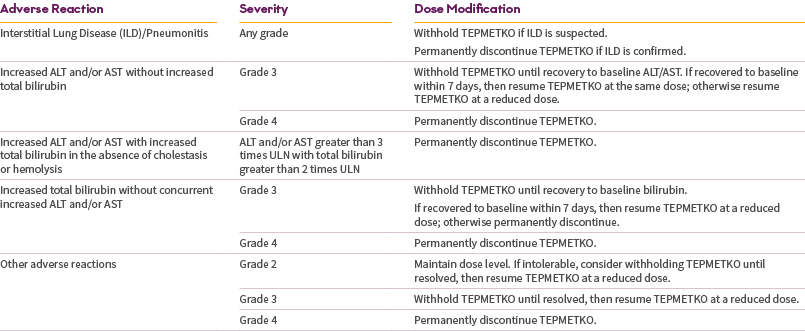
Flexible dose adjustment
Dose Reduction for the Management of ARs: 225 mg
- Permanently discontinue in patients who are unable to tolerate the 225 mg once-daily dose.
- Management of some ARs may require temporary interruption or permanent discontinuation. See the full Prescribing Information for recommended dose modifications of TEPMETKO.
One Dose.
Once a Day.
Patient selection
Select patients for treatment with TEPMETKO based on the presence of METex14 skipping alterations in plasma or tumor specimens. Testing for the presence of METex14 skipping alterations in plasma specimens is recommended only in patients for whom a tumor biopsy cannot be obtained. If an alteration is not detected in a plasma specimen, reevaluate the feasibility of biopsy for tumor tissue testing. An FDA-approved test for detection of METex14 skipping alterations in NSCLC for selecting patients for treatment with TEPMETKO is not available.
Administration to patients who have difficulty swallowing solids
- Place TEPMETKO tablet(s) in a glass containing 30 mL (1 ounce) of non-carbonated water. No other liquids should be used or added.
- Stir, without crushing, until the tablet(s) is dispersed into small pieces (tablets will not completely dissolve) and drink immediately or within 1 hour.
- Swallow the tablet dispersion. Do not chew pieces of the tablet.
- Rinse the glass with an additional 30 mL and drink immediately ensuring no residue remains in the glass and the full dose is administered.
If an administration via a naso-gastric tube (with at least 8 French gauge) is required, disperse the tablet(s) in 30 mL of non-carbonated water as described above.
- Administer the 30 mL of liquid immediately or within 1 hour as per naso-gastric tube manufacturer’s instructions.
- Immediately rinse twice with 30 mL each time to ensure that no residue remains in the glass or syringe and the full dose is administered.
Storage and handling
TEPMETKO (tepotinib) tablets: 225 mg tepotinib, white-pink, oval, biconvex film-coated tablet with embossment “M” on one side and plain on the other side.

The blister cards consist of a child-resistant blister foil.
Store TEPMETKO at 20°C-25°C (68°F-77°F); excursions permitted to 15°C-30°C (59°F-86°F) [see USP-NF Controlled Room Temperature]. Store in original package.
AR=adverse reaction; MET=mesenchymal-epithelial transition; METex14+=mesenchymal-epithelial transition exon 14 skipping alterations; mNSCLC=metastatic non-small cell lung cancer.
IMPORTANT SAFETY INFORMATION
TEPMETKO can cause interstitial lung disease (ILD)/pneumonitis, which can be fatal. Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis (eg, dyspnea, cough, fever). Immediately withhold TEPMETKO in patients with suspected ILD/pneumonitis and permanently discontinue if no other potential causes of ILD/pneumonitis are identified. ILD/pneumonitis occurred in 2% of patients treated with TEPMETKO, with one patient experiencing a Grade 3 or higher event; this event resulted in death.
TEPMETKO can cause hepatotoxicity, which can be fatal. Monitor liver function tests (including alanine aminotransferase [ALT], aspartate aminotransferase [AST], and total bilirubin) prior to the start of TEPMETKO, every 2 weeks during the first 3 months of treatment, then once a month or as clinically indicated, with more frequent testing in patients who develop increased transaminases or total bilirubin. Based on the severity of the adverse reaction, withhold, dose reduce, or permanently discontinue TEPMETKO. Increased ALT/increased AST occurred in 18% of patients treated with TEPMETKO. Grade 3 or 4 increased ALT/AST occurred in 4.7% of patients. A fatal adverse reaction of hepatic failure occurred in one patient (0.2%). The median time-to-onset of Grade 3 or higher increased ALT/AST was 47 days (range 1 to 262).
TEPMETKO can cause pancreatic toxicity in the form of elevations in amylase and lipase levels. Increased amylase and/or lipase occurred in 13% of patients, with Grade 3 and 4 increases occurring in 5% and 1.2% of patients, respectively. Monitor amylase and lipase levels at baseline and regularly during treatment with TEPMETKO and temporarily withhold, dose reduce, or permanently discontinue based on severity of the adverse event.
TEPMETKO can cause embryo-fetal toxicity. Based on findings in animal studies and its mechanism of action, TEPMETKO can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential or males with female partners of reproductive potential to use effective contraception during treatment with TEPMETKO and for one week after the last dose.
Avoid concomitant use of TEPMETKO with certain P-gp substrates where minimal concentration changes may lead to serious or life-threatening toxicities. If concomitant use is unavoidable, reduce the P-gp substrate dosage if recommended in its approved product labeling.
Fatal adverse reactions occurred in one patient (0.3%) due to pneumonitis, one patient (0.3%) due to hepatic failure, one patient (0.3%) due to dyspnea from fluid overload, one patient (0.3%) due to pneumonia, one patient (0.3%) due to sepsis, and one patient (0.3%) from unknown cause.
Serious adverse reactions occurred in 51% of patients who received TEPMETKO. Serious adverse reactions in >2% of patients included pleural effusion (6%), pneumonia (6%), edema (5%), general health deterioration (3.8%), dyspnea (3.5%), musculoskeletal pain (2.9%), and pulmonary embolism (2.2%).
The most common adverse reactions (≥20%) in patients who received TEPMETKO were edema (81%), nausea (31%), fatigue (30%), musculoskeletal pain (30%), diarrhea (29%), dyspnea (24%), rash (21%), and decreased appetite (21%).
Clinically relevant adverse reactions in <10% of patients who received TEPMETKO included ILD/pneumonitis, fever, dizziness, pruritus, and headache.
Selected laboratory abnormalities (≥20%) from baseline in patients receiving TEPMETKO in descending order were: decreased albumin (81%), increased creatinine (60%), decreased lymphocytes (57%), increased alkaline phosphatase (ALP) (52%), increased ALT (50%), increased AST (40%), decreased sodium (36%), decreased hemoglobin (31%), increased gamma-glutamyltransferase (GGT) (29%), increased potassium (26%), increased amylase (25%), decreased leukocytes (25%), decreased platelets (24%), and increased lipase (21%).
The most common Grade 3-4 laboratory abnormalities (≥2%) in descending order were: decreased lymphocytes (15%), decreased albumin (9%), decreased sodium (9%), increased GGT (6%), increased amylase (5%), increased lipase (5%), increased ALT (4.9%), increased AST (3.6%), and decreased hemoglobin (3.6%).
INDICATION
TEPMETKO is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) harboring mesenchymal-epithelial transition (MET) exon 14 skipping alterations.
Please see the full Prescribing Information for TEPMETKO.
Reference: 1. TEPMETKO® (tepotinib) [prescribing information]. EMD Serono, Inc., Boston, MA.