This site is intended for US healthcare professionals only.
METex14 skipping should be identified at diagnosis as it plays an important role in NSCLC oncogenesis.1,2
METex14 matters
 met-matters
met-matters
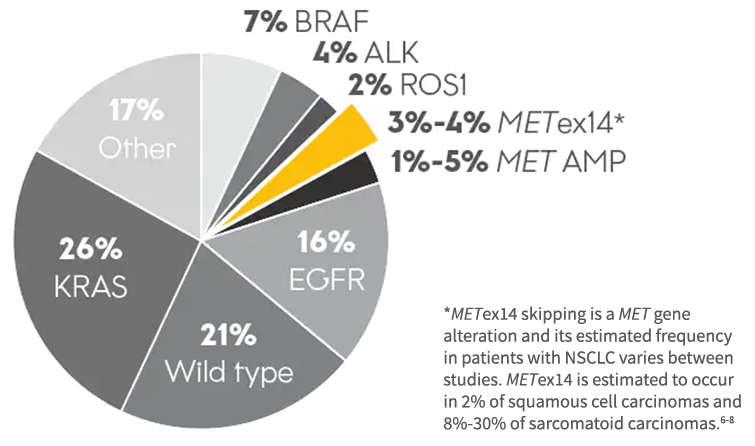
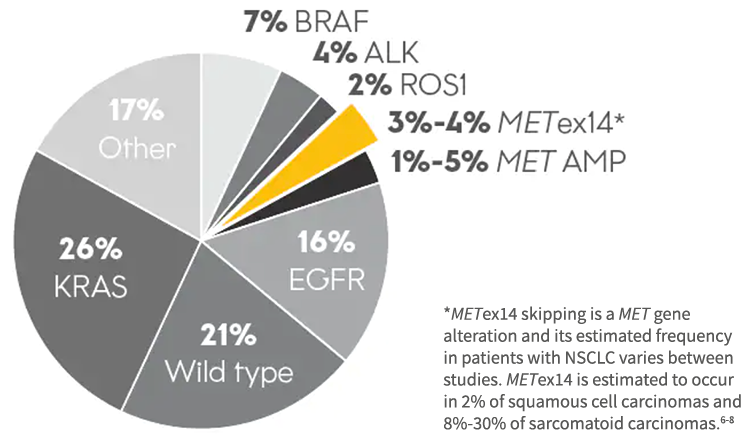
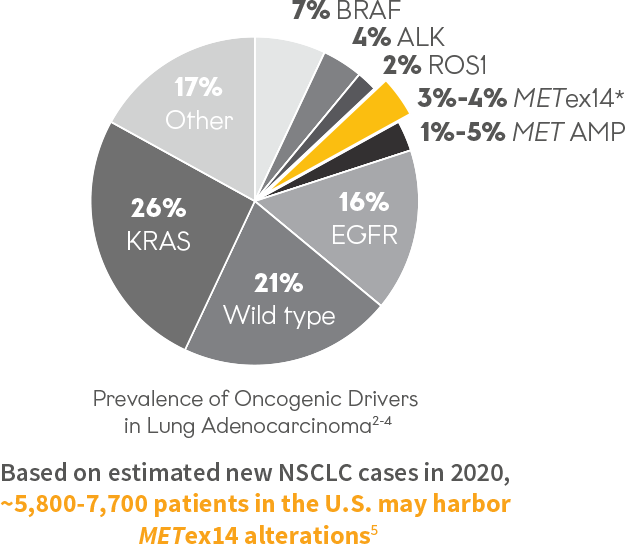
Prevalence of Oncogenic Drivers in Lung Adenocarcinoma2-4
Based on 2023 estimates,
~5,700 to ~8,100 patients with NSCLC harboring METex14 alterations may be diagnosed each year in the US⁵
Based on 2023 estimates, ~5,700 to ~8,100 patients with NSCLC harboring METex14 alterations may be diagnosed each year in the US⁵
*METex14 skipping is a MET gene alteration and its estimated frequency in patients with NSCLC varies between studies. METex14 is estimated to occur in 2% of squamous cell carcinomas and 8%-30% of sarcomatoid carcinomas.6-8
3%-4% of patients may harbor METex14 skipping alterations2
Patients with METex14+ skipping alterations have been associated with having advanced disease and a poor prognosis.1
These patients tend to be older than patients with other oncogenic drivers (54-65 years of age in ALK, ROS1, EGFR, and KRAS) with an average age of ~74 years at diagnosis.7
~65% of patients with METex14 skipping alterations may be PD-L1 positive ( ≥1% expression)9,10
It is important to test for this primary oncogenic driver to identify patients at diagnosis and help inform treatment decisions1,2
NCCN
RECOMMENDS
- Clinicians obtain molecular testing results for actionable biomarkers in eligible patients with metastatic NSCLC before administering first-line ICI therapy ± chemotherapy, if clinically feasible†
- Targeted therapies for patients with metastatic NSCLC and specific oncogenic drivers independent of PD-L1 levels
- For patients who must immediately start therapy but molecular testing is pending, consider holding immunotherapy for one cycle, unless no driver mutations are confirmed present
†The NCCN Guidelines® for NSCLC provide recommendations for certain individual biomarkers that should be tested and recommend testing techniques but do not endorse any specific commercially available biomarker assays or commercial laboratories.
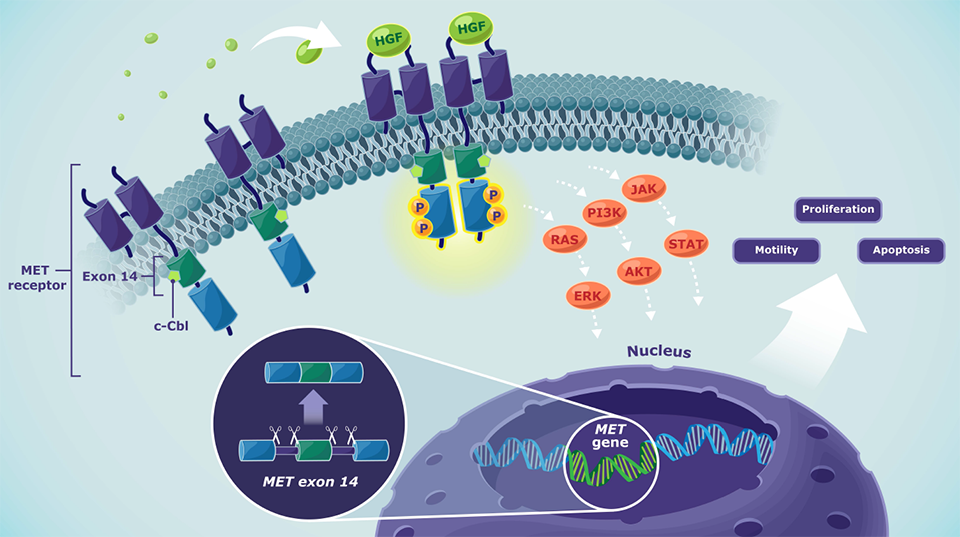
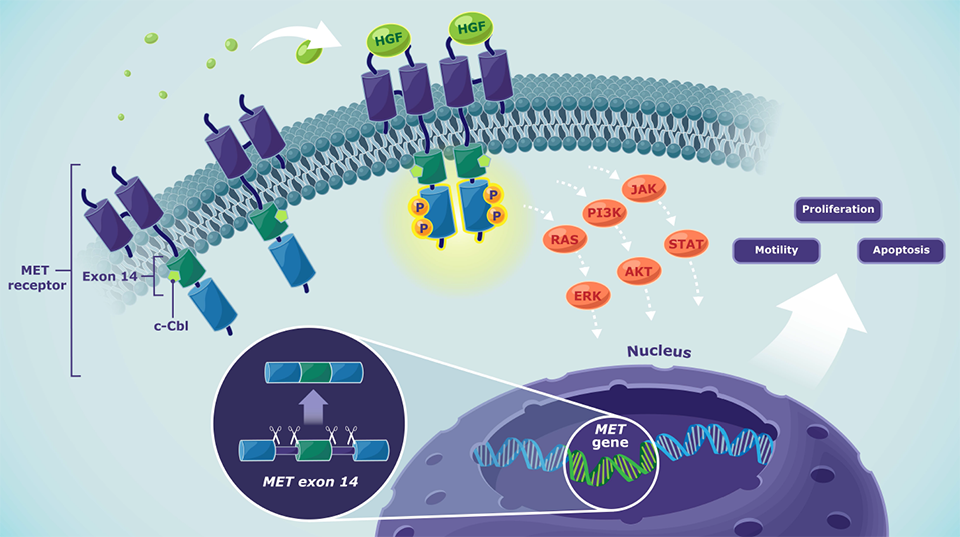
MET pathway
Normal MET pathway signaling4
- Depending on the cellular context, activated MET signaling may induce processes including cell proliferation, motility, and apoptosis
- MET alterations like METex14 skipping or MET amplification lead to a dysregulation of MET signaling
- Dysregulated MET signaling may lead to tumor cell proliferation, migration, and survival
 met-pathway
met-pathway
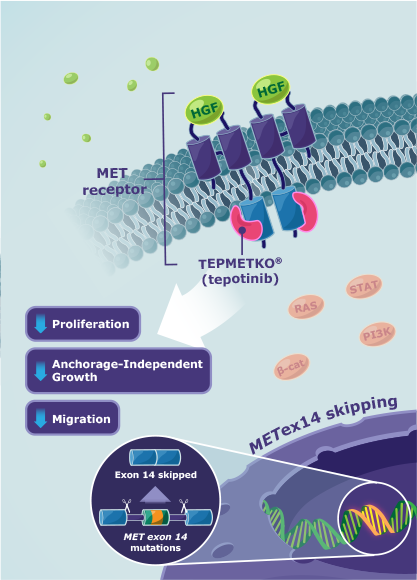
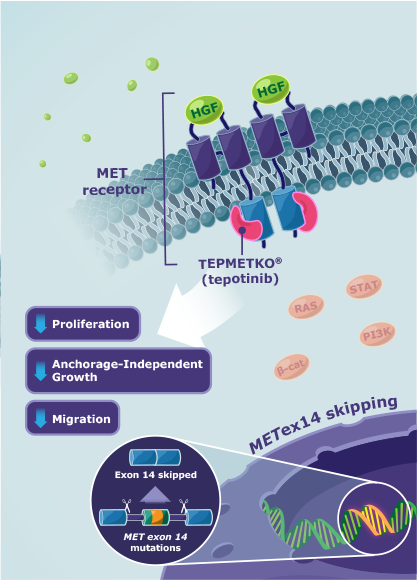
Mechanism of action
TEPMETKO® (tepotinib) MOA12
TEPMETKO is an inhibitor that targets MET tyrosine kinase activity, including aberrant activity observed with METex14 skipping alterations.
- TEPMETKO inhibits hepatocyte growth factor (HGF)-dependent and ‑independent MET phosphorylation and MET-dependent downstream signaling pathways
- TEPMETKO also inhibits melatonin 2 and imidazoline 1 receptors at clinically achievable concentrations
- In vitro, TEPMETKO inhibits tumor cell proliferation, anchorage-independent growth, and migration of MET-dependent tumor cells
In animal models with oncogenic activation of MET, including METex14 skipping alterations, TEPMETKO inhibited tumor growth, led to sustained inhibition of MET phosphorylation, and, in one model, decreased the formation of metastases.
 met-moa
met-moa
AKT=Ak strain transforming; ALK=anaplastic lymphoma kinase; β-cat=β-catenin; BRAF=v-raf murine sarcoma viral oncogene homolog B1; c-Cbl=casitas B lineage lymphoma; EGFR=epidermal growth factor receptor; ERK=extracellular signal-regulated kinase; HGF=hepatocyte growth factor; JAK=Janus kinase; KRAS=Kirsten rat sarcoma virus; MET=mesenchymal-epithelial transition; MET AMP= mesenchymal-epithelial transition amplification; METex14=mesenchymal-epithelial transition exon 14; NSCLC=non-small cell lung cancer; P=phosphorylation; PD-L1=programmed death-ligand 1; PI3K=phosphoinositide 3-kinase; RAS=rat sarcoma virus; ROS1=reactive oxygen species proto-oncogene 1; STAT=signal transducer and activator of transcription.
IMPORTANT SAFETY INFORMATION
TEPMETKO can cause interstitial lung disease (ILD)/pneumonitis, which can be fatal. Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis (eg, dyspnea, cough, fever). Immediately withhold TEPMETKO in patients with suspected ILD/pneumonitis and permanently discontinue if no other potential causes of ILD/pneumonitis are identified. ILD/pneumonitis occurred in 2% of patients treated with TEPMETKO, with one patient experiencing a Grade 3 or higher event; this event resulted in death.
TEPMETKO can cause hepatotoxicity, which can be fatal. Monitor liver function tests (including alanine aminotransferase [ALT], aspartate aminotransferase [AST], and total bilirubin) prior to the start of TEPMETKO, every 2 weeks during the first 3 months of treatment, then once a month or as clinically indicated, with more frequent testing in patients who develop increased transaminases or total bilirubin. Based on the severity of the adverse reaction, withhold, dose reduce, or permanently discontinue TEPMETKO. Increased ALT/increased AST occurred in 18% of patients treated with TEPMETKO. Grade 3 or 4 increased ALT/AST occurred in 4.7% of patients. A fatal adverse reaction of hepatic failure occurred in one patient (0.2%). The median time-to-onset of Grade 3 or higher increased ALT/AST was 47 days (range 1 to 262).
TEPMETKO can cause pancreatic toxicity in the form of elevations in amylase and lipase levels. Increased amylase and/or lipase occurred in 13% of patients, with Grade 3 and 4 increases occurring in 5% and 1.2% of patients, respectively. Monitor amylase and lipase levels at baseline and regularly during treatment with TEPMETKO and temporarily withhold, dose reduce, or permanently discontinue based on severity of the adverse event.
TEPMETKO can cause embryo-fetal toxicity. Based on findings in animal studies and its mechanism of action, TEPMETKO can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential or males with female partners of reproductive potential to use effective contraception during treatment with TEPMETKO and for one week after the last dose.
Avoid concomitant use of TEPMETKO with certain P-gp substrates where minimal concentration changes may lead to serious or life-threatening toxicities. If concomitant use is unavoidable, reduce the P-gp substrate dosage if recommended in its approved product labeling.
Fatal adverse reactions occurred in one patient (0.3%) due to pneumonitis, one patient (0.3%) due to hepatic failure, one patient (0.3%) due to dyspnea from fluid overload, one patient (0.3%) due to pneumonia, one patient (0.3%) due to sepsis, and one patient (0.3%) from unknown cause.
Serious adverse reactions occurred in 51% of patients who received TEPMETKO. Serious adverse reactions in >2% of patients included pleural effusion (6%), pneumonia (6%), edema (5%), general health deterioration (3.8%), dyspnea (3.5%), musculoskeletal pain (2.9%), and pulmonary embolism (2.2%).
The most common adverse reactions (≥20%) in patients who received TEPMETKO were edema (81%), nausea (31%), fatigue (30%), musculoskeletal pain (30%), diarrhea (29%), dyspnea (24%), rash (21%), and decreased appetite (21%).
Clinically relevant adverse reactions in <10% of patients who received TEPMETKO included ILD/pneumonitis, fever, dizziness, pruritus, and headache.
Selected laboratory abnormalities (≥20%) from baseline in patients receiving TEPMETKO in descending order were: decreased albumin (81%), increased creatinine (60%), decreased lymphocytes (57%), increased alkaline phosphatase (ALP) (52%), increased ALT (50%), increased AST (40%), decreased sodium (36%), decreased hemoglobin (31%), increased gamma-glutamyltransferase (GGT) (29%), increased potassium (26%), increased amylase (25%), decreased leukocytes (25%), decreased platelets (24%), and increased lipase (21%).
The most common Grade 3-4 laboratory abnormalities (≥2%) in descending order were: decreased lymphocytes (15%), decreased albumin (9%), decreased sodium (9%), increased GGT (6%), increased amylase (5%), increased lipase (5%), increased ALT (4.9%), increased AST (3.6%), and decreased hemoglobin (3.6%).
INDICATION
TEPMETKO is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) harboring mesenchymal-epithelial transition (MET) exon 14 skipping alterations.
Please see the full Prescribing Information for TEPMETKO.
References: 1. Awad MM, Leonardi GC, Kravets S, et al. Impact of MET inhibitors on survival among patients with non-small cell lung cancer harboring MET exon 14 mutations: a retrospective analysis. Lung Cancer. 2019;133:96-102. doi:10.1016/j.lungcan.2019.05.011 2. Salgia R. MET in lung cancer: biomarker selection based on scientific rationale. Mol Cancer Ther. 2017;16(4):555-565. doi:10.1158/1535-7163.MCT-16-0472 3. Rosell R, Karachaliou N. Large-scale screening for somatic mutations in lung cancer. Lancet. 2016;387(10026):1354-1356. doi:10.1016/S0140-6736(15)01125-3 4. Drilon A, Cappuzzo F, Ou SHI, Camidge DR. Targeting MET in lung cancer: will expectations finally be MET? J Thorac Oncol. 2017;12(1):15-26. doi:10.1016/j.jtho.2016.10.014 5. American Cancer Society. Key statistics for lung cancer. Accessed September 14, 2023. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html 6. Schrock AB, Frampton GM, Suh J, et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol. 2016;11(9):1493-1502. doi:10.1016/j.jtho.2016.06.004 7. Tong JH, Yeung SF, Chan AWH, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22(12):3048-3056. doi:10.1158/1078-0432.CCR-15-2061 8. Liu X, Jia Y, Stoopler MB, et al. Next-generation sequencing of pulmonary sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. J Clin Oncol. 2016;34(8):794-802. 9. Pruis MA, Geurts-Giele WRR, von der TJH, et al. Highly accurate DNA-based detection and treatment results of MET exon 14 skipping mutations in lung cancer. Lung Cancer. 2020;140:46-54. doi:10.1016/j.lungcan.2019.11.010 10. Sabari JK, Leonardi GC, Shu CA, et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol. 2018;29(10):2085-2091. doi:10.1093/annonc/mdy334 11. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed February 27, 2024. To view the most recent and complete version of the guidelines, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 12. TEPMETKO® (tepotinib) [prescribing information]. EMD Serono, Inc., Boston, MA.